The endoplasmic reticulum (ER) is a large, membrane-bound organelle in eukaryotic cells that plays a crucial role in protein and lipid synthesis, folding, and transport. As one of the most essential organelles, the ER operates like a factory, coordinating biochemical reactions, and serves as a transportation network for the cell.
Historical Background
The endoplasmic reticulum was discovered in the 1940s by American scientists Keith Porter, Albert Claude, and Ernest Fullam, who used an electron microscope to study cell structure in greater detail. The term “endoplasmic reticulum” was first introduced by Porter in 1953 to describe this membrane-bound network within the cell’s cytoplasm. This discovery represented a significant milestone in cell biology, as it revealed the existence of a highly organized internal membrane system distinct from the cell membrane.
As cell biology advanced, scientists recognized that the ER was not just a structural component but a critical site for protein synthesis and other vital functions. The realization that the ER comes in two forms—rough and smooth—added further insights into its specialized roles within cells.
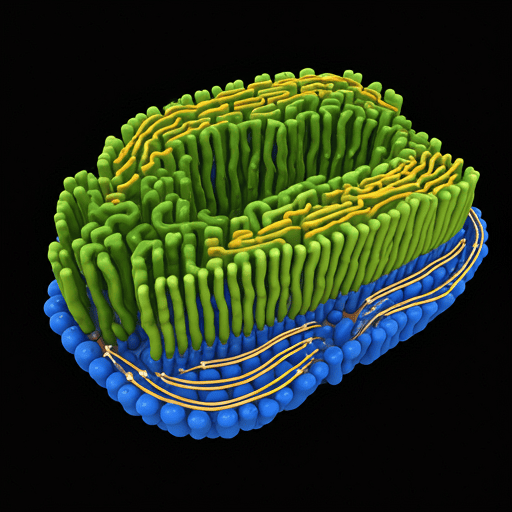
Structure of the Endoplasmic Reticulum
The ER is a continuous membrane system connected to the nuclear envelope and spread throughout the cytoplasm. It has a complex network of tubules, vesicles, and flattened sacs called cisternae. The structure of the ER can be categorized into two primary types, each with distinct physical and functional characteristics:
- Rough Endoplasmic Reticulum (RER): The RER is named for its rough appearance due to ribosomes attached to its cytoplasmic surface. These ribosomes are sites for protein synthesis, and their presence indicates the RER’s primary role in producing proteins, especially those destined for secretion or insertion into cell membranes.
- Smooth Endoplasmic Reticulum (SER): Unlike the RER, the SER lacks ribosomes, giving it a smooth appearance. The SER is primarily involved in lipid and carbohydrate metabolism, detoxification of drugs and toxins, and calcium storage, depending on the cell type.
Example: In liver cells, the SER is abundant and specialized in detoxifying various drugs and chemicals, while in muscle cells, it forms a sarcoplasmic reticulum to store and release calcium, essential for muscle contraction.
Types and Functions of the Endoplasmic Reticulum
The ER’s multifunctional nature supports a wide range of cellular activities. Here’s a closer look at the key functions of the RER and SER:
A. Rough Endoplasmic Reticulum (RER)
- Protein Synthesis: The RER’s ribosomes assemble amino acids into polypeptide chains, which are then folded and modified into functional proteins. This process is especially crucial for proteins that are secreted outside the cell, incorporated into the cell membrane, or sent to other organelles.
- Protein Folding and Quality Control: Proteins synthesized in the RER undergo folding and post-translational modifications, such as glycosylation (adding carbohydrate groups). Chaperone proteins in the ER help fold proteins correctly, while misfolded proteins are targeted for degradation.
- Example: In pancreatic cells, the RER is abundant to support the synthesis of insulin and digestive enzymes that are later secreted into the bloodstream and gastrointestinal tract.
B. Smooth Endoplasmic Reticulum (SER)
- Lipid and Steroid Synthesis: The SER is responsible for producing phospholipids, cholesterol, and steroid hormones. These lipids are essential for cellular membranes and hormone production, especially in cells of the adrenal glands and gonads.
- Detoxification: In liver cells, the SER contains enzymes that detoxify harmful substances like drugs and metabolic waste products. These enzymes add hydroxyl groups to toxins, making them more water-soluble and easier to excrete.
- Calcium Storage and Release: The SER regulates calcium storage and release, crucial for cell signaling pathways. In muscle cells, the specialized sarcoplasmic reticulum releases calcium ions to trigger muscle contraction.
- Example: In adrenal gland cells, the SER is abundant for the synthesis of steroid hormones such as cortisol and aldosterone, which are critical for metabolism and electrolyte balance.
ER-Golgi Transport and the Secretory Pathway
The ER is closely connected with the Golgi apparatus, forming a transport network for synthesized proteins and lipids. Newly synthesized proteins in the RER are packaged into vesicles that bud off and are transported to the Golgi, where they undergo further processing and sorting. The Golgi then directs these proteins to their final destinations within or outside the cell.
Example: In immune cells, antibodies are synthesized in the ER, transported to the Golgi for glycosylation, and then secreted into the bloodstream to help fight infections.
Modern Research and Advances in Understanding the ER
Recent advances in molecular biology, imaging technologies, and genomics have deepened our understanding of the ER’s functions and its involvement in health and disease. Key areas of modern research include:
A. Unfolded Protein Response (UPR) and ER Stress
One area of intense research is the ER’s role in the unfolded protein response (UPR), a cellular stress response triggered when misfolded proteins accumulate in the ER. UPR activation helps restore protein-folding balance, but if stress persists, it can lead to cell death. Chronic ER stress is linked to various diseases, including neurodegenerative disorders, diabetes, and cancer.
- Example: In diseases like Alzheimer’s and Parkinson’s, the accumulation of misfolded proteins in the ER and the resultant ER stress contribute to the death of neurons.
B. ER’s Role in Metabolic Diseases
The ER’s lipid metabolism functions are crucial for maintaining energy balance. Researchers are studying the SER’s role in metabolic conditions like obesity, insulin resistance, and non-alcoholic fatty liver disease. Disruptions in lipid synthesis or ER stress in liver cells have been shown to affect metabolic regulation.
C. ER and Immunity
The ER plays a role in immune response by synthesizing proteins that are critical to immune signaling and inflammation. For example, the ER processes antigens for presentation on the cell surface, helping immune cells recognize foreign substances.
- Example: In response to infection, immune cells use the ER to produce large amounts of cytokines, signaling molecules that aid in immune response coordination.
D. ER and Cancer Research
ER stress and the UPR have been implicated in cancer cell survival and resistance to treatment. Some cancer cells exploit UPR signaling to cope with stress from rapid growth and avoid apoptosis. Targeting the ER stress pathway has become a promising area of cancer research, with potential for developing treatments that selectively induce ER stress in cancer cells.
Future Directions and Technological Advances
Technological advancements in live-cell imaging, super-resolution microscopy, and single-cell RNA sequencing are helping scientists better understand ER dynamics. These tools allow real-time observation of ER functions and how the ER interacts with other organelles under different cellular conditions.
Gene Editing and Synthetic Biology Approaches
Using CRISPR gene editing, researchers can modify ER-associated genes to study their effects on cell function and disease development. Synthetic biology approaches are also being explored to engineer artificial ER systems that mimic natural ER processes, providing a controlled environment to study complex biochemical pathways.
References
- Alberts, B., et al.Molecular Biology of the Cell. 6th Edition, Garland Science, 2015.
- Porter, K. R., Claude, A., & Fullam, E. F. “A Study of Tissue Culture Cells by Electron Microscopy: Methods and Preliminary Observations.” Journal of Experimental Medicine, 81(3), 233-246, 1945.
- Ron, D., & Walter, P. “Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response.” Nature Reviews Molecular Cell Biology, 8, 519-529, 2007.
- Schwarz, D. S., & Blower, M. D. “The Endoplasmic Reticulum: Structure, Function, and Response to Cellular Signaling.” Cellular Signalling, 38, 79-92, 2017.
- Hetz, C., & Saxena, S. “ER Stress and the Unfolded Protein Response in Neurodegeneration.” Nature Reviews Neurology, 13(8), 477-491, 2017.
- Xu, C., Bailly-Maitre, B., & Reed, J. C. “Endoplasmic Reticulum Stress: Cell Life and Death Decisions.” Journal of Clinical Investigation, 115(10), 2656-2664, 2005.
- Görlach, A., Klappa, P., & Kietzmann, T. “The Endoplasmic Reticulum: Folding, Calcium Homeostasis, Signaling, and Redox Control.” Antioxidants & Redox Signaling, 8(9-10), 1391-1418, 2006.
- Schwarzbaum, P. J., & Gilfillan, S. “Role of the Endoplasmic Reticulum in Protein Processing and Lipid Synthesis.” International Journal of Biochemistry & Cell Biology, 124, 105778, 2020.
