In 1666, Sir Isaac Newton discovered that when a beam of sunlight is passed through a glass prism, the emerging beam of light is not white but consists instead of a continuous spectrum of colors ranging from violet at one end to red at the other As Fig. 1 shows, the range of colors we perceive in visible light represents a very small portion of the electromagnetic spectrum. On one end of the spectrum are radio waves with wavelengths billions of times longer than those of visible light. On the other end of the spectrum are gamma rays with wavelengths millions of times smaller than those of visible light. The electromagnetic spectrum can be expressed in terms of wavelength, frequency, or energy.
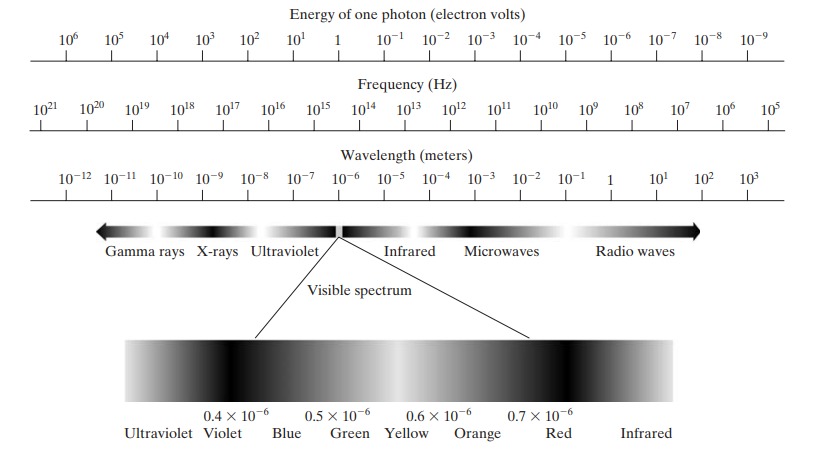
Light, a form of electromagnetic radiation, is central to our understanding of the physical universe. The electromagnetic spectrum encompasses a broad range of electromagnetic waves, from the very short wavelengths of gamma rays to the exceedingly long wavelengths of radio waves. This note delves into the fundamental mathematical concepts underpinning the nature of light and the electromagnetic spectrum.
The Nature of Electromagnetic Radiation
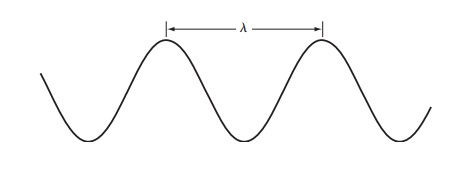
Electromagnetic radiation can be described as a wave that carries energy through space or matter. These waves are characterized by their wavelength (), frequency (), and speed (). The speed of light in a vacuum is a fundamental constant of nature, approximately meters per second.
Key Equation:
This equation illustrates the inverse relationship between wavelength and frequency: as the wavelength increases, the frequency decreases, and vice versa.
Energy of Electromagnetic Waves
The energy () of a photon, a quantum of light, is directly proportional to its frequency and can be calculated using Planck’s equation:
where is Planck’s constant ( Joule·seconds).
Example:
To find the energy of a photon with a frequency of Hz, we use:
The Electromagnetic Spectrum
The electromagnetic spectrum is divided into several regions, based on wavelength and frequency. These include (from longest wavelength to shortest): radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
- Radio Waves:
- Microwaves:
- Infrared:
- Visible Light:
- Ultraviolet:
- X-rays:
- Gamma Rays:
Visible Spectrum and Color Perception
The visible spectrum is a small part of the electromagnetic spectrum observable by the human eye, ranging approximately from 380 nm (violet) to 750 nm (red). The color perceived is determined by the wavelength of the light.
Applications and Implications
The electromagnetic spectrum has myriad applications across science and technology. For instance, radio waves are used for communication, infrared for thermal imaging, and X-rays for medical diagnostics.
Challenges and Future Directions
Understanding and manipulating the electromagnetic spectrum poses both opportunities and challenges, particularly in terms of resolution and detection capabilities. As technology advances, new methods of harnessing electromagnetic waves for communication, medicine, and science are continually being explored.
The study of light and the electromagnetic spectrum is a fascinating blend of physics and mathematics, revealing the intricate balance of wavelength, frequency, and energy that defines the behavior of electromagnetic waves. Through mathematical equations and principles, we can understand and apply the vast potential of the electromagnetic spectrum across various domains, driving forward innovations in technology and science.
Reference
- Digital Image Processing, Third Edition Rafael C. Gonzalez
